Page subtitle will go here.
STUDENTS
Page subheading will go here in this space.
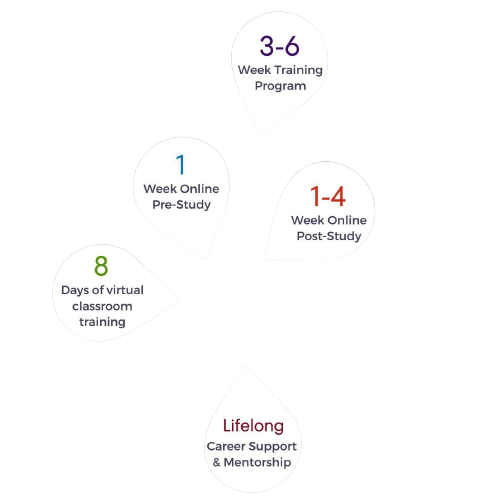
Paragraph sample text goes here. The Clinical Research Fastrack Bootcamp Training Program is a highly intensive, accelerated course, designed to prepare new professionals for the field of Clinical Research in 3-6 weeks. This immersive training offers foundational instruction in ICH GCP, FDA regulations, the Informed Consent process, and every other core competency of clinical trial facilitation. Course instructors are active directors, managers, CRAs, and specialists from leading Clinical Research institutions across the U.S.
Clinical Research Fastrack is a trusted education provider for hundreds of the top research sites and our graduates are getting hired in record numbers, nationwide.
We offer a wide variety of scholarships and financing options to recruit the highest quality candidates who will end up working in Clinical Research facilities.
Topic Subheading Here
Paragraph sample text goes here. The Clinical Research Fastrack Bootcamp Training Program is a highly intensive, accelerated course, designed to prepare new professionals for the field of Clinical Research in 3-6 weeks. This immersive training offers foundational instruction in ICH.
Topic Subheading Here
Paragraph sample text goes here. The Clinical Research Fastrack Bootcamp Training Program is a highly intensive, accelerated course, designed to prepare new professionals for the field of Clinical Research in 3-6 weeks. This immersive training offers foundational instruction in ICH.
Topic Subheading Here
Paragraph sample text goes here. The Clinical Research Fastrack Bootcamp Training Program is a highly intensive, accelerated course, designed to prepare new professionals for the field of Clinical Research in 3-6 weeks. This immersive training offers foundational instruction in ICH.
Informative statement about videowill go here.
Subheading 2
Subheading 3
- 8-Day Classroom Training (Currently held via Zoom)
- Additional Course Materials and Online Learning
- Career Coaching & Job Assistance
- Clinical Experience Externship (Optional)
Clinical Research Fastrack is a trusted education provider for
hundreds of the top research sites and our graduates are
getting hired in record numbers, nationwide.
We offer a wide variety of scholarships and financing options
to recruit the highest quality candidates who will end up
working in Clinical Research facilities.
Subheading 3
- Program Overview
- CURRICULUM BREAKDOWN:
- 8-Day Classroom Training (Currently held via Zoom)
- Additional Course Materials and Online Learning
- Career Coaching & Job Assistance
- Clinical Experience Externship (Optional)
- TOPICS INCLUDE
- Research Concepts
- ICH GCP Guidelines & Protecting the Rights of the Patient
- FDA Regulations
- Regulatory Affairs
- Dissection of a Protocol & Clinical Trial Budgeting
- Good Documentation Practices
- Recruitment & Retention of Clinical Research Subjects
- Investigator Responsibilities
- Monitoring, Quality Assurance, & Inspections
- Data Management Systems
- Clinical Trial Operations
- The Informed Consent Process
- Research Skills & Research Communications Training
- Tying It All Together
- Clinical Research Communication and Career Opportunities
- Clinical Research Fastrack Individualized Career Coaching
Subheading 2
Subheading 3
- 8-Day Classroom Training (Currently held via Zoom)
- Additional Course Materials and Online Learning
- Career Coaching & Job Assistance
- Clinical Experience Externship (Optional)
Clinical Research Fastrack is a trusted education provider for
hundreds of the top research sites and our graduates are
getting hired in record numbers, nationwide.
We offer a wide variety of scholarships and financing options
to recruit the highest quality candidates who will end up
working in Clinical Research facilities.
Subheading 3
- Program Overview
- CURRICULUM BREAKDOWN:
- 8-Day Classroom Training (Currently held via Zoom)
- Additional Course Materials and Online Learning
- Career Coaching & Job Assistance
- Clinical Experience Externship (Optional)
- TOPICS INCLUDE
- Research Concepts
- ICH GCP Guidelines & Protecting the Rights of the Patient
- FDA Regulations
- Regulatory Affairs
- Dissection of a Protocol & Clinical Trial Budgeting
- Good Documentation Practices
- Recruitment & Retention of Clinical Research Subjects
- Investigator Responsibilities
- Monitoring, Quality Assurance, & Inspections
- Data Management Systems
- Clinical Trial Operations
- The Informed Consent Process
- Research Skills & Research Communications Training
- Tying It All Together
- Clinical Research Communication and Career Opportunities
- Clinical Research Fastrack Individualized Career Coaching
Subheading 3
The Clinical Research Fastrack program is a fast-paced, intensive learning course. During the 8-day, online classroom portion of the program, students spend ten hours a day in the online classroom, fully engaging with the content that builds their educational foundation in this field.
Subheading 3
After completing the externship portion of the course, students have the opportunity to work with our Clinical Research Fastrack Directors and utilize our expertise throughout the process of applying to jobs in the Clinical Research field. Our graduate services department offers support with networking, outreach to clinical research organizations, resume and interview prep, and application strategies to help our graduates find the best positions available.

Subheading 3
The Clinical Research Fastrack program is a fast-paced, intensive learning course. During the 8-day, online classroom portion of the program, students spend ten hours a day in the online classroom, fully engaging with the content that builds their educational foundation in this field.
Subheading 3
After completing the externship portion of the course, students have the opportunity to work with our Clinical Research Fastrack Directors and utilize our expertise throughout the process of applying to jobs in the Clinical Research field. Our graduate services department offers support with networking, outreach to clinical research organizations, resume and interview prep, and application strategies to help our graduates find the best positions available.
Subheading 3
After completing the classroom training, a student will have the option to be placed in an externship at one of our partnering research sites, helping to run active clinical trials. Here, the student gains hands-on experience supporting the research team at the site. This opportunity provides the chance to be involved in every step of the trial process, as well as to perform several “mock trials” in order to cover all aspects of the curriculum.
Interested in applying?
Connect with one of our
admissions team members to
apply for an upcoming course.
